

This is another great way to learn the order of the elements if you do well hearing instead of (or in addition to) seeing. Make nonsense words made from element symbols.This works well if you learn better by hearing information rather than seeing it on paper. There is a popular one called We Just Crammed the Table, which is set to a Billy Joel tune. You can learn a song someone else created or make up your own. Walk away, come back, and review old material, add a new group, walk away, etc. So learn a section of the table, go off and do something else, write out what you learned in that first section, and try to learn a new section. This involves repeated practice and exposure. To truly commit the periodic table to memory, you need to access the part of your brain responsible for long-term memory. Cramming might serve for short-term memorization, like for a test the very next day, but you won't remember anything a few days later. You'll remember the table much better if you spread out the memorization process over multiple sessions instead of cramming the entire table at once. Rather than attempting to memorize all of the elements at once, learn one group at a time, master that group, and then learn the next group until you know the whole table. It may be helpful to view an ordered list of the elements. You could memorize element groups (different color groups), go one row at a time, or memorize in sets of 20 elements. How you memorize the table depends on what works best for you and your learning style, but here are some recommendations that may help: The periodic table as a list of elements arranged so as to demonstrate trends in their physical and chemical properties.Once you have the table, you need to learn it.Describe and model the structure of the atom in terms of the nucleus, protons, neutrons and electrons comparing mass and charge of protons neutrond and electrons. Use the Periodic Table to predict the ratio of atoms in compounds of two elements. Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.
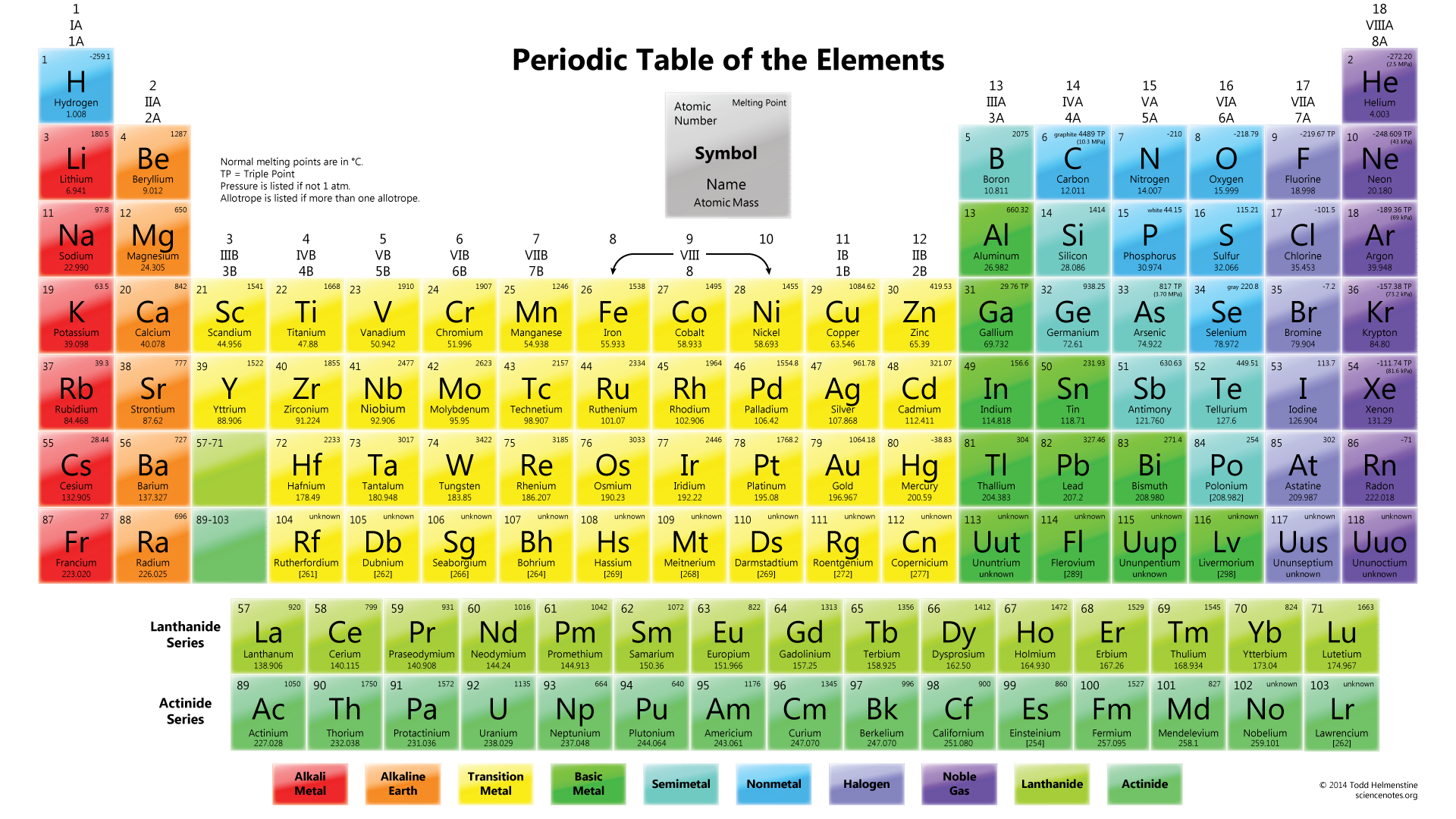
Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.(g) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.2.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.(h) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.1.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES.(a) elements being arranged according to atomic number in the Periodic Table.Unit 1: THE LANGUAGE OF CHEMISTRY, STRUCTURE OF MATTER AND SIMPLE REACTIONS.The Periodic Table can be used to determine whether an element is a metal or non-metal.Atomic structure and bonding related to properties of materials.Elements are arranged in the periodic table in order of increasing atomic number.RSC Yusuf Hamied Inspirational Science Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
